
Metalyse - Administration |
Metalyse 25 mg is now available for adults for thrombolytic treatment of acute ischaemic stroke within 4.5 hours from last known well and after exclusion of intracranial haemorrhage1
Because Every
Minute Matters

Single IV bolus administration
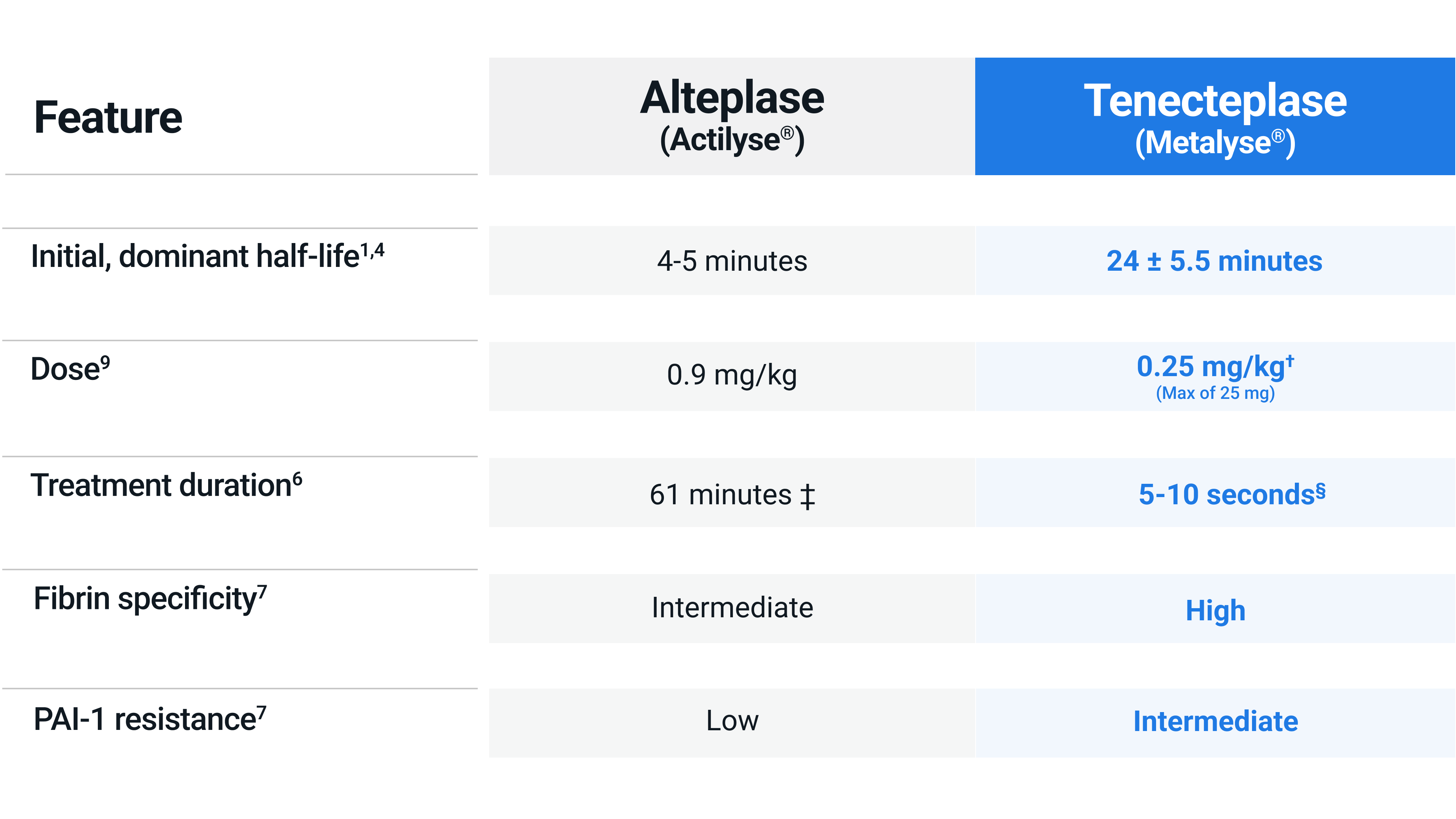
Metalyse® 25 mg is administered by a single IV bolus over 5 to 10 seconds, eliminating the need for a one-hour infusion, as required for Actilyse®2-3
Metalyse® 25 mg comes as one single vial of powder for reconstitution
The dosing for Metalyse® 25 mg is with 5 weight-based tiers:
| Weight (kg) | Corresponding volume of reconstituted solution (ml) | Tenecteplase (Units) | Tenecteplase (mg) |
| < 60 | 3 | 3000 | 15 |
| ≥ 60 to < 70 | 3.5 | 3500 | 17.5 |
| ≥ 70 to < 80 | 4 | 4000 | 20 |
| ≥ 80 to <90 | 4.5 | 4500 | 22.5 |
| ≥ 90 | 5 | 5000 | 25 |
Why is Metalyse® 25 mg quicker and easier to use compared to Actilyse®?
Tenecteplase is a genetically modified version of alteplase. Mutations in the protein sequence increased the specificity of Metalyse® (tenecteplase) to fibrin and made it more resistant to natural inhibitors, which increased its half-life and allowed for shorter treatment duration.1,4
Footnotes
-
†
Tenecteplase was administrated within 4.5 hours after onset of stroke symptoms, as a one-time decile-weight-tiered bolus dose, based on 0.25 mg/kg by 10 kg steps, for the maximum weight at each tier: < 60 kg, 15 mg tenecteplase; ≥ 60 to < 70 kg, 17.5 mg; ≥ 70 to < 80 kg, 20 mg; ≥ 80 to < 90 kg, 22.5 mg; and ≥ 90 kg, 25 mg.
-
‡
10% bolus over 1 minute, the remainer infused over 60 minutes.6
-
§
One time 5 second bolus.6
-
Actilyse (alteplase) indication: Fibrinolytic treatment of acute ischaemic stroke (within 4,5 hours of last know well and after exclusion of intracerebral haemorhage by suitable imaging technology)
References
-
Metalyse® European Summary of Product Characteristics.
-
Menon BK, et al. Lancet 2022; 400:161-169.
-
Bivard A, et al. Lancet Neurol. 2022; 21:520-27.
-
Actilyse® European Summary of Product Characteristics.
-
Dancsecs K. A. et al. Am J Emerg Med. 2021; 47:90-94.
-
Miller, SE and Warach, SJ. Neurotherapeutics 2023; 20:664–678.
-
Zhu A, et al. Res Pract Thromb Haemost. 2022; 6:e12795.
